
Design Name:
Iodine Clock Reaction
Design Description:
A clock reaction using starch, potassium iodide, sodium thiosulfate, acetic acid, and hydrogen peroxide. A solution of hydrogen peroxide is mixed with another solution with the rest of the chemicals, and the reaction will begin. After some time, the solution will undergo a drastic colour change. This reaction is used as a clock for the car, to determine how long the motors will run. Data is collected in the lab, and a model is created to predict ideal concentrations from time of reaction.
The experiment that will be performed is the design of a stopping mechanism based on the thiosulfate variant of the iodine clock reaction. The reactions that occur are as follows:
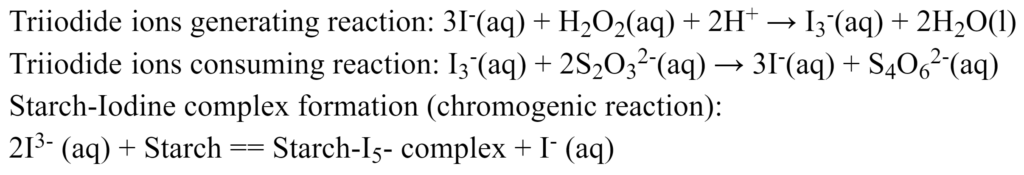
When the reaction first begins, the reaction mixture remains colourless as not enough triiodide (I3–) has formed to form the purple starch-Iodine complex. As the limiting reagent is Sulfite ion, once it is depleted, there will be a build of Triiodide in the reaction mixture. This will lead to the sudden colour change that is required to trigger the photodetector and thus the stopping mechanism.
There are two components to the stopping mechanism: the stirring mechanism and the reaction vessel. The reaction vessel will contain the reaction mixture and makes sure that no spillages will occur. The reaction vessel is made from ABS plastic, which will not react with any of the reactants used or any of the products produced. The stirring mechanism is to ensure that all of the reaction components are well mixed and that the reactants don’t separate from each other. The stirring mechanism will be composed of either two permanent neodymium magnets attached to a motor or a stationary alternating electromagnet. Attached to the reaction vessel will be a photodetector, which will detect the sudden color change of the reaction mixture when the reaction is complete. The photodetector is linked to the main circuit and cuts the power that links to the car’s motor.
In the lab, the reaction takes place inside a small beaker (100mL minimum) which is placed on top of a hot plate that can operate a stir bar. A separate hotplate is used to heat up a starch solution to ensure that the starch dissolves in the water.
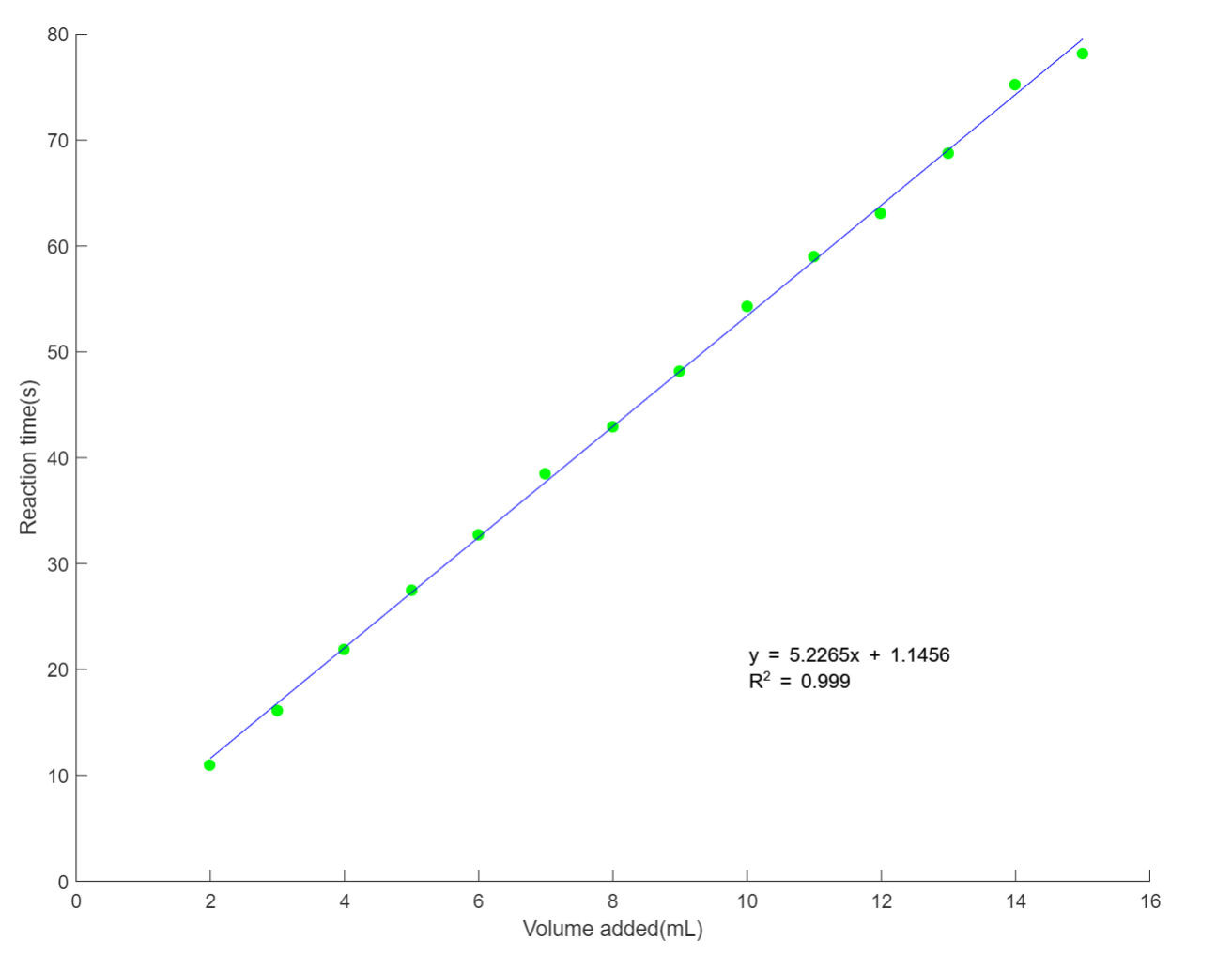
The team mainly focuses on finding the most accurate method to controll the reaction time. By controlling the amount of the limiting reagent, the reaction time can be precisely controlled
Active Team Members:
Jhoselyn Jaramillo, Yuqi Zhou, Xingyu Feng, Rulangyue Zhao, Fariha, Olevia Pal, Natasha Pereira, Reuvin Sunny, Xinyu Zhang, Andrew Chin, Ryan Zhang, Shih Rong Pan, Mingfei Liu, Paola Calle
